Novartis Wins FDA Approval of Kisqali for Early Breast Cancer
Novartis NVS announced that the FDA has approved breast cancer drug Kisqali (ribociclib) for a broader population.
The regulatory body approved Kisqali in combination with an aromatase inhibitor for the adjuvant treatment of people with hormone receptor-positive/human epidermal growth factor receptor 2-negative (HR+/HER2-) stage II and III early breast cancer (EBC) at high risk of recurrence, including those with node-negative (N0) disease.
Kisqali is a selective cyclin-dependent kinase inhibitor, a class of drugs that helps slow the progression of cancer by inhibiting two proteins — cyclin-dependent kinase 4 and 6 (CDK4/6).
The broad indication in HR+/HER2- stage II and III EBC at high risk of recurrence approximately doubles the population eligible for CDK4/6 inhibitor adjuvant therapy.
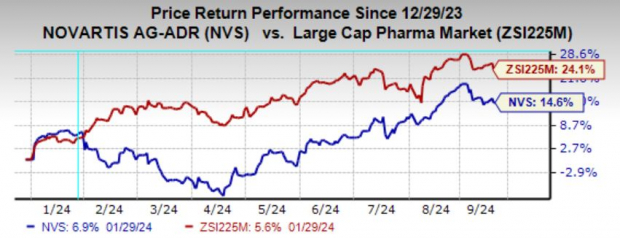
Year to date, shares of Novartis have risen 14.6% compared with the industry’s growth of 24.1%.

Image Source: Zacks Investment Research
Broader Label for NVS’ Kisqali
The FDA approval is based on strong results from the late-stage NATALEE trial. The results showed a significant and clinically meaningful 25.1% reduction in risk of disease recurrence in a broad population of patients with HR+/HER2- stage II and III EBC treated with adjuvant Kisqali plus endocrine therapy compared to ET alone, including those with high-risk N0 disease.
The invasive disease-free survival benefit was consistently observed across all patient subgroups.
Please note that Kisqali is already approved for the treatment of metastatic breast cancer (MBC) in several countries.
The latest FDA approval of Kisqali for this early breast cancer population, including those with N0 disease, should enable NVS to offer treatment with a CDK4/6 inhibitor to a significantly broader group of patients.
NVS recently presented an updated analysis from the NATALEE trial at the European Society for Medical Oncology Congress 2024. Results showed that Kisqali caused a deepening benefit beyond the three-year treatment period and reduced the risk of recurrence by 28.5% compared to ET alone, in patients with stage II and III HR+/HER2- EBC.
Novartis will continue to evaluate NATALEE patients for long-term outcomes, including overall survival.
Kisqali: A Top Drug for NVS
Kisqali is one of the key growth drivers for NVS, which is now a pure-play innovative medicine company with a focus on core therapeutic areas — cardiovascular, renal and metabolic, immunology, neuroscience and oncology.
It is one of the leading breast cancer drugs in the United States and outside the country, with a dominant market share. The drug generated sales worth $1.3 billion in the first half of 2024.
Approximately 90% of breast cancer cases in the United States are diagnosed early (stages I-III). These patients remain at risk of cancer recurrence (in most cases as an incurable metastatic disease).
The drug’s approval for a broader population should further fuel sales.
Regulatory reviews for Kisqali as an EBC treatment are ongoing worldwide, including in the EU and China.
Last year, the FDA also expanded Eli Lilly’s LLY Verzenio’s (abemaciclib) indication. Verzenio, a CDK4/6 inhibitor, was approved in combination with ET for the adjuvant treatment of HR+HER2-, node-positive, EBC at a high risk of recurrence.
Eli Lilly is witnessing a stupendous run in 2024, riding high on the success of its GLP-1 drugs — Mounjaro and Zepbound.
NVS’ Zacks Rank & A Stock to Consider
NVS currently carries a Zacks Rank #3 (Hold).
A better-ranked stock from the large-cap pharma industry is Pfizer, which currently sports a Zacks Rank #2 (Buy).
Estimates for PFE’s 2024 earnings have risen from $2.39 to $2.62 per share over the past 60 days. For 2025, the bottom-line estimate has risen from $2.75 to $2.85 over the same time frame.
Market News and Data brought to you by Benzinga APIs
© 2024 Benzinga.com. Benzinga does not provide investment advice. All rights reserved.

Leave a Reply