BHVN Stock Up as Rare Neurological Disease Study Meets Primary Goal
Shares of Biohaven Ltd. BHVN were up 13.7% on Sept. 23 after the company announced positive top-line data from the pivotal BHV4157-206-RWE study, which evaluated its pipeline candidate, troriluzole, for the treatment of spinocerebellar ataxia (SCA), a rare and debilitating neurodegenerative disease.
Currently, there are no FDA-approved therapy for the given indication.
The study showed the efficacy of troriluzole on the mean change from baseline in the modified functional Scale for the Assessment and Rating of Ataxia (f-SARA) following three years of treatment.
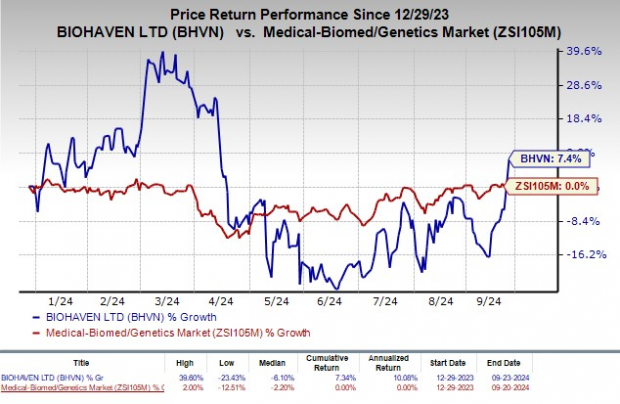
Year to date, shares of Biohaven have gained 7.4% with no change for the industry.

Image Source: Zacks Investment Research
BHVN’s Pivotal BHV4157-206-RWE Study Data
The study met the primary endpoint on the change from baseline in the f-SARA at three years in all study population genotypes following treatment with troriluzole (200 mg) in SCA patients.
Also, treatment with troriluzole led to statistically significant improvements in the f-SARA at years one and two. Data collected from multiple analyses showed a robust and clinically meaningful slowing of disease progression in SCA patients.
Importantly, treatment with troriluzole slowed disease progression by 50% to 70%, representing a 1.5-2.2 years delay in disease progression over three years compared to untreated patients.
Treatment with troriluzole demonstrated statistically significant superiority on nine consecutive, prespecified primary and secondary endpoints of the study.
Per management, troriluzole is the first treatment to show a delay in disease progression for SCA patients.
BHVN’s Upcoming Plans for Troriluzole
Based on the positive data from the BHV4157-206-RWE study, along with previous safety and efficacy data, BHVN plans to submit a new drug application (NDA) for troriluzole in SCA with the FDA in the fourth quarter of 2024.
If approved, the company plans to launch troriluzole in the United States in 2025.
The FDA has granted Fast-Track and orphan drug designation to troriluzole for treating SCA. The European Medicines Agency has also granted orphan drug designation to troriluzole for treating SCA.
As a result of the orphan drug and fast-track designations granted by the FDA, the NDA for troriluzole is eligible for a priority review.
Troriluzole is also being evaluated in two late-stage studies for treating obsessive-compulsive disorder (OCD). Interim analysis of the second phase III OCD study is expected later in 2024, while top-line data from the first phase III OCD study is anticipated in the first half of 2025.
Zacks Rank & Stocks to Consider
Biohaven currently carries a Zacks Rank #3 (Hold).
Some better-ranked stocks in the biotech sector are ANI Pharmaceuticals, Inc. ANIP, Krystal Biotech, Inc. KRYS and Fulcrum Therapeutics, Inc. FULC, each sporting a Zacks Rank #1 (Strong Buy) at present.
In the past 60 days, estimates for ANI Pharmaceuticals’ 2024 earnings per share have moved up from $4.49 to $4.82. Earnings per share estimates for 2025 have improved from $5.51 to $5.93. Year to date, shares of ANIP have gained 9%.
ANIP’s earnings beat estimates in each of the trailing four quarters, with the average surprise being 31.32%.
In the past 60 days, estimates for Krystal Biotech’s 2024 earnings per share have increased from $2.09 to $2.38. Earnings per share estimates for 2025 have improved from $4.33 to $7.31. Year to date, shares of KRYS have risen 45.3%.
KRYS’ earnings beat estimates in three of the trailing four quarters while missing on the remaining occasion, with the average surprise being 45.95%.
In the past 60 days, estimates for Fulcrum Therapeutics’ 2024 loss per share have narrowed from $1.33 to 28 cents. Loss per share estimates for 2025 have narrowed from $1.71 to $1.14. Year to date, shares of FULC have plunged 52.6%.
FULC’s earnings beat estimates in each of the trailing four quarters, with the average surprise being 393.18%.
© 2024 Benzinga.com. Benzinga does not provide investment advice. All rights reserved.

Leave a Reply