AstraZeneca Inks Agreement to Boost Cardiovascular Pipeline
AstraZeneca AZN announced that it has signed an exclusive license agreement with China-based CSPC Pharmaceutical Group Ltd for developing an early-stage, small molecule Lipoprotein (a) (Lp(a)) disruptor for helping patients with dyslipidaemia.
The latest agreement is likely to strengthen AstraZeneca’s cardiovascular pipeline, which is being studied for treating various cardiovascular diseases.
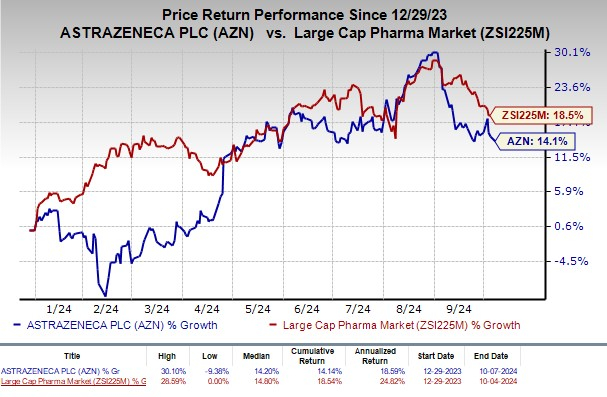
Year to date, shares of AstraZeneca have rallied 14.1% compared with the industry’s rise of 18.5%.

Image Source: Zacks Investment Research
More on AZN’s Latest Agreement
Per the deal, AZN will make an upfront payment of $100 million to CSPC. The company is also entitled to pay up to $1.92 billion for further development and commercialization milestones plus tiered royalties to CSPC.
Per the latest agreement with CSPC, AstraZeneca will gain access to the latter’s pre-clinical candidate, YS2302018, an oral Lp(a) disruptor, and develop the same for various cardiovascular diseases. The company will develop YS2302018 alone or in combination studies, including with the oral small molecule PCSK9 inhibitor, AZD0780.
Discovered by CSPC, YS2302018 has been shown to effectively prevent the formation of Lp(a), a form of low-density lipoprotein that plays a key role in transporting cholesterol in the bloodstream.
Per the company, the addition of YS2302018 to AZN’s cardiovascular pipeline might help patients to more effectively manage their dyslipidaemia and cardiometabolic diseases.
AZN’s Airsupra Meet Goal in BATURA Study
In a separate press release, AstraZeneca announced positive data from the phase IIIb BATURA study, which evaluated its pressurized metered-dose inhaler (pMDI), Airsupra (albuterol/budesonide).
Data from the study showed that treatment with Airsupra led to a statistically significant and clinically meaningful reduction in the risk of a severe exacerbation when used as an as-needed rescue medication in response to symptoms versus as-needed albuterol in patients with intermittent or mild persistent asthma. This was the primary endpoint of the BATURA study.
Airsupra is approved for as-needed treatment or prevention of bronchoconstriction and to reduce the risk of exacerbations in people with asthma aged 18 years and above in the United States. It is the first and only anti-inflammatory rescue medication for the given condition.
AZN’s Zacks Rank & Other Stocks to Consider
AstraZeneca currently carries a Zacks Rank #2 (Buy).
Some other top-ranked stocks in the biotech sector are ANI Pharmaceuticals, Inc. ANIP, ADMA Biologics, Inc. ADMA and Alnylam Pharmaceuticals, Inc. ALNY, each sporting a Zacks Rank #1 (Strong Buy) at present. In the past 60 days, estimates for ANI Pharmaceuticals’ 2024 earnings per share have moved up from $4.53 to $4.81. Earnings per share estimates for 2025 have improved from $5.38 to $5.86. Year to date, shares of ANIP have increased 1.3%.
ANIP’s earnings beat estimates in each of the trailing four quarters, with the average surprise being 31.32%.
In the past 60 days, estimates for ADMA Biologics’ 2024 earnings per share have increased from 35 cents to 49 cents. Earnings per share estimates for 2025 have improved from 53 cents to 64 cents. Year to date, shares of ADMA have surged 346.5%.
ADMA’s earnings beat estimates in each of the trailing four quarters, with the average surprise being 105.63%.
In the past 60 days, estimates for Alnylam’s 2024 loss per share have narrowed from $1.20 to 63 cents. Loss per share estimates for 2025 have narrowed from 34 cents to 27 cents. Year to date, shares of ALNY have rallied 39.4%.
ALNY’s earnings beat estimates in each of the trailing four quarters, with the average surprise being 108.53%.
Market News and Data brought to you by Benzinga APIs
© 2024 Benzinga.com. Benzinga does not provide investment advice. All rights reserved.

Leave a Reply