Trump Secures Key Swing State North Carolina, Extending Lead Over Harris In 2024 Presidential Race
Former President Donald Trump won North Carolina in the 2024 presidential race, securing the state’s 16 electoral votes with a substantial lead over Vice President Kamala Harris.
What Happened: With nearly 94% of expected votes counted, Trump led by more than 195,000 votes or 3.6 percentage points, according to projections by the Associated Press. The margin represents a stronger performance than his 2020 victory in North Carolina when he won by less than 2 percentage points.
Election officials reported higher voter turnout in areas where Trump performed well while noting decreased participation in districts favoring Harris. The pattern marks a significant shift from previous voting trends in the state.
The announcement of Trump’s North Carolina victory sparked immediate celebration at his election watch party. Supporters erupted in cheers, with some pumping their fists in the air and others jumping in excitement as the results appeared on screen. The crowd began chanting “fight, fight, fight” as the AP projection was confirmed.
See Also: Bitcoin Past $73K — Dollar, Gold, Stock Futures Rise As Trump-Harris Results Trickle In
The prediction market Polymarket, which has processed more than $3.5 billion in election-related trading volume, showed significant movement following the North Carolina results.
Current market sentiment reflects Trump at 97% and Harris at 3.4% in presidential odds. The platform’s traders strongly anticipate that the Associated Press will make its final election call by Wednesday, November 6, assigning this outcome an 82% probability.
The race for the presidency continues as votes are being counted in other states across the country. North Carolina’s results represent just one step in the path to securing the 270 electoral votes needed to win the presidency.
Read Next:
Image Via Flickr
Disclaimer: This content was partially produced with the help of AI tools and was reviewed and published by Benzinga editors.
Market News and Data brought to you by Benzinga APIs
© 2024 Benzinga.com. Benzinga does not provide investment advice. All rights reserved.
Grand Opening: The Greater Danbury, CT Area's Largest Self-Storage Facility
PURCHASE, N.Y., Nov. 5, 2024 /PRNewswire/ — dSA Capital LLC and Extra Space Storage are excited to announce the grand opening of its newest self-storage facility in Brookfield, CT, located less than half a mile from the Danbury city limits. The state-of-the-art, four-story building spans 110,000 square feet, making it the largest single-building self-storage facility in the greater Danbury area.
Conveniently situated at the bustling intersection of Candle Lake Road, Federal Road, and Grays Bridge Road, the facility is easily accessible via Exit 11 on Super Route 7. The area is a prominent retail hub, home to major national brands such as Costco, Home Depot, Raymour & Flanigan, BJ’s, and Kohl’s, as well as Stew Leonard’s and the newly opened Big Y supermarket.
The facility features 810 storage units, including 57 highly desirable drive-up units, with sizes up to 10′ x 30′. It is climate-controlled, ensuring cool temperatures in the summer and warmth in the winter. The building’s exterior design is modern and visually appealing, with soft gray and white facades accented by Extra Space’s signature wasabi green panels, trim, and overhead doors.
Extra Space Storage, a publicly traded REIT managing over 3,800 facilities nationwide, will brand, operate, and manage this location. Visit extraspace.com to learn more about renting a unit at this property.
The development team is led by de Stefanis & Associates of White Plains, NY. Founded in 1958 and currently headed by President Carl de Stefanis, P.E., the company has a long history of successful projects, including warehouses, office buildings, and banking facilities in the New York City metropolitan area.
CONTACT: Carl de Stefanis – carl.de.stefanis@de-stefanis.com
![]() View original content to download multimedia:https://www.prnewswire.com/news-releases/grand-opening-the-greater-danbury-ct-areas-largest-self-storage-facility-302296934.html
View original content to download multimedia:https://www.prnewswire.com/news-releases/grand-opening-the-greater-danbury-ct-areas-largest-self-storage-facility-302296934.html
SOURCE dSA Capital LLC
Market News and Data brought to you by Benzinga APIs
© 2024 Benzinga.com. Benzinga does not provide investment advice. All rights reserved.
Electric Hoist Market Size to Hit USD 1.9 Billion by 2031, Growing at 5.8% CAGR Amid Rising Demand Across Key Industries: Transparency Market Research Inc.
Wilmington, Delaware, United States, Transparency Market Research, Inc., Nov. 05, 2024 (GLOBE NEWSWIRE) — The global electric hoist market (سوق الرافعات الكهربائية) is estimated to flourish at a CAGR of 5.8% from 2023 to 2031. Transparency Market Research projects that the overall sales revenue for electric hoist is estimated to reach US$ 1.9 billion by the end of 2031.
The circular economy is a potential driver in the market. Companies are increasingly adopting refurbishment and remanufacturing practices, reconditioning and modernizing existing electric hoists. This approach aligns with sustainability goals, reducing waste while meeting performance standards.
Get Sample PDF Research Report with Latest Industry Insights @ https://www.transparencymarketresearch.com/sample/sample.php?flag=S&rep_id=55332
Electric Hoist Market: Competitive Landscape
The electric hoist market shows fierce competition led by prominent players such as Konecranes, Columbus McKinnon Corporation, and Street Crane Company Limited. These industry giants dominate with a diverse portfolio of electric hoists catering to industrial, construction, and commercial sectors.
Companies like Ingersoll Rand and Demag Cranes AG (now part of Konecranes) intensify competition with their innovative hoisting solutions. Regional players, including GIS AG and VERLINDE, further contribute, emphasizing niche market expertise.
This competitive landscape thrives on product innovation, technological advancements, and a focus on customized solutions, driving the electric hoist market forward amidst growing demand for efficient and reliable lifting equipment across various industries. Some prominent manufacturers are as follows:
- Tianjing Kunda Hoisting Equipment Co. Ltd
- IMER International SpA
- ABUS Kransysteme GmbH
- Street Crane Company Limited
- Ingersoll Rand
- Konecranes
- Columbus McKinnon Corporation
- Hitachi Industrial Equipment Systems Co. Ltd
- Kran Direkt GmbH & Co. KG
- Columbus McKinnon Corporation
The resurgence of local manufacturing initiatives presents a lucrative opportunity. Amid global supply chain disruptions, many industries prioritize localized production to reduce dependency and enhance agility. This trend encourages the establishment of smaller-scale manufacturing facilities, fostering a demand for electric hoists used in production and assembly lines.
The advent of predictive maintenance technologies subtly influences market growth. Implementation of predictive analytics and condition monitoring systems in electric hoists allows proactive maintenance, reducing downtime and repair costs. This emerging driver emphasizes the importance of minimizing operational disruptions, elevating the reliability and longevity of hoisting equipment.
Key Findings of the Market Report
- Wire or rope hoist dominates the electric hoist market due to its versatility, ability to handle heavier loads, and diverse applications.
- The capacity segment of “above 16000 lbs” leads the electric hoist market due to demand for heavy lifting applications.
- Construction leads the electric hoist market, demanding reliable lifting solutions for material handling and heavy load operations on job sites.
Electric Hoist Market Growth Drivers & Trends
- Increasing automation in manufacturing and logistics drives demand for electric hoists, enhancing operational efficiency and streamlining material handling processes.
- Rising construction activities globally fuel the need for electric hoists for lifting heavy loads and materials at construction sites.
- Emphasis on workplace safety and ergonomic design in electric hoists drives product innovation, ensuring operator comfort and accident prevention.
- Integration of smart features such as IoT connectivity and remote monitoring elevates electric hoist capabilities, optimizing performance and maintenance.
- Expansion of renewable energy projects necessitates electric hoists for lifting and transporting heavy components in wind farms and solar installations.
Download Sample Copy of this Report @ https://www.transparencymarketresearch.com/sample/sample.php?flag=S&rep_id=55332
Global Electric Hoist Market: Regional Profile
- North America leads the market due to extensive industrialization and infrastructure development. Key players like Columbus McKinnon Corporation and Harrington Hoists, Inc. dominate, offering advanced electric hoists catering to manufacturing, construction, and automotive sectors. Stringent safety regulations further drive market growth, ensuring the adoption of high-quality hoisting solutions.
- In Europe, a robust manufacturing sector and emphasis on automation fuel the demand for electric hoists. Street Crane Company Limited and Konecranes hold prominence, delivering innovative lifting solutions, focusing on efficiency and safety in various industries.
- The Asia Pacific emerges as a rapidly expanding market, driven by flourishing manufacturing hubs in countries like China and India. Players such as GIS AG and VERLINDE capitalize on this growth, providing cost-effective electric hoists tailored to diverse applications. As industrial activities surge, the region remains pivotal in shaping the global Electric Hoist Market through innovation and market expansion.
Product Portfolio
- SStreet Crane Company Limited offers a comprehensive product portfolio specializing in crane systems and lifting solutions. Their range includes overhead cranes, hoists, and components, catering to diverse industrial sectors, emphasizing quality, innovation, and tailored lifting solutions for varying applications and industries.
- Ingersoll Rand showcases a diverse product line comprising air compressors, power tools, and material handling equipment. Their offerings cater to industrial, commercial, and residential sectors, focusing on efficiency, reliability, and sustainability in providing innovative solutions for diverse applications.
- Konecranes presents an extensive portfolio of lifting equipment and services, encompassing overhead cranes, port solutions, and automated material handling systems. Their offerings cater to various industries, prioritizing safety, efficiency, and advanced technology in delivering comprehensive lifting solutions and maintenance services.
Electric Hoist Market: Key Segments
By Hoist Rope Type
- Chain hoist
- Wire or Rope Hoist
By Capacity
- Up to 1000 lbs
- 1000 lbs to 2000 lbs
- 2000 lbs to 4000 lbs
- 4000 lbs to 6000 lbs
- 6000 lbs to 8000 lbs
- 8000 lbs to 10000 lbs
- 10000 lbs to 12000 lbs
- 12000 lbs to 16000 lbs
- Above 16000 lbs
By End-Use Industry
- Automotive & Railway
- Aerospace & Defense
- Transportation & Logistics
- Construction
- Shipping & Marine
- Material Handling
- Agriculture & Forestry
- Mining
- Oil & Gas
- Others (Entertainment, Waste Management, etc.)
By Application
- Commercial Recovery
- Cranes
- Fixed Cranes
- Mobile Cranes
- Workboats
- Utility
- Others (Military recovery, Stage maker, etc.)
By Distribution Channel
- Direct Sales
- Indirect Sales
By Region
- North America
- Europe
- Asia Pacific
- Middle East & Africa
- South America
Buy this Premium Research Report: https://www.transparencymarketresearch.com/checkout.php?rep_id=55332<ype=S
More Trending Reports by Transparency Market Research –
- Multilayer pipes market– The global multilayer pipes market (سوق الأنابيب متعددة الطبقات) is expected to reach US$ 1.05 Billion by the end of 2031 and it is estimated to grow at a CAGR of 6.4% from 2022 to 2031.
- Power Tiller Market– The global power tiller market (سوق محراث الطاقة) is expected to reach US$ 33.2 Billion by the end of 2031 and it is estimated to grow at a CAGR of 5.9% from 2022 to 2031.
- North America Meteorological Equipment Market – The North America meteorological equipment market is estimated to grow at a CAGR of 7.1% from 2024 to 2034 and reach US$ 10.0 Billion by the end of 2034.
- Asia-Pacific PCB Compression Molding Press Market – The Asia-Pacific PCB compression molding press market is estimated to grow at a CAGR of 3.2% from 2023 to 2031 and reach US$ 51.9 Million by the end of 2031.
About Transparency Market Research
Transparency Market Research, a global market research company registered at Wilmington, Delaware, United States, provides custom research and consulting services. Our exclusive blend of quantitative forecasting and trends analysis provides forward-looking insights for thousands of decision makers. Our experienced team of Analysts, Researchers, and Consultants use proprietary data sources and various tools & techniques to gather and analyses information.
Our data repository is continuously updated and revised by a team of research experts, so that it always reflects the latest trends and information. With a broad research and analysis capability, Transparency Market Research employs rigorous primary and secondary research techniques in developing distinctive data sets and research material for business reports.
Contact:
Transparency Market Research Inc.
CORPORATE HEADQUARTER DOWNTOWN,
1000 N. West Street,
Suite 1200, Wilmington, Delaware 19801 USA
Tel: +1-518-618-1030
USA – Canada Toll Free: 866-552-3453
Website: https://www.transparencymarketresearch.com
Email: sales@transparencymarketresearch.com
Follow Us: LinkedIn| Twitter| Blog | YouTube

Market News and Data brought to you by Benzinga APIs
© 2024 Benzinga.com. Benzinga does not provide investment advice. All rights reserved.
Press Release: Dupixent approved in the EU as the first and only medicine for young children with eosinophilic esophagitis
Dupixent approved in the EU as the first and only medicine for young children with eosinophilic esophagitis
- Approval based on phase 3 data showing significantly more children aged one to 11 years on Dupixent achieved histological disease remission at 16 weeks compared to placebo, which was sustained up to one year
- Dupixent is the first-ever medicine in the EU indicated to treat these young patients, who persistently struggle to eat at a critical stage in life where growth is crucial
Paris and Tarrytown, NY, November 6, 2024. The European Medicines Agency has approved Dupixent (dupilumab) to treat eosinophilic esophagitis (EoE) in children as young as one year of age. Specifically, the approval covers children aged one to 11 years who weigh at least 15 kg and who are inadequately controlled by, intolerant to, or who are not candidates for conventional medicinal therapy. This expands the initial approval in the European Union (EU) for EoE in adults and adolescents and makes Dupixent the first and only medicine indicated to treat these young patients. Dupixent is also approved in this young age group in the US and Canada.
Roberta Giodice
President, ESEO Italia
“Young children with eosinophilic esophagitis are at the beginning of their life-long journey with a disease that challenges their ability to eat. Parents of these children have often relied on restrictive diets that do not specifically address the disease and can stunt their growth at a critical time in development that could impact them for years to come. We are pleased that research continues and offers new treatment options to improve the quality of their care.”
Houman Ashrafian, MD, PhD
Executive Vice President, Head of Research and Development, Sanofi
“Up to half of all children in the EU with eosinophilic esophagitis remain uncontrolled despite existing standard of care treatment options, and, as a result, many of these young patients struggle to maintain weight due to serious symptoms such as difficulty swallowing and vomiting. This milestone provides an important new treatment for pediatric patients who were previously without options specifically approved for their disease. With this novel approach to addressing an underlying cause of eosinophilic esophagitis, Dupixent has the potential to give these young children a better chance to thrive.”
The approval is based on the two-part (Part A and B) EoE KIDS phase 3 study in children aged one to 11 years, which established a bridge showing the response to Dupixent in children with EoE is similar to that of the approved adult and adolescent populations. In Part A, children who received a higher dose of Dupixent (n=37) based on a weight-based dosing regimen experienced the following outcomes, compared to placebo (n=34) at 16 weeks:
- 68% achieved histological disease remission (≤6 eosinophils/high power field) compared to 3%, the primary endpoint. These results were sustained for up to one year in Part B of the study.
- 86% reduction in peak esophageal intraepithelial eosinophil count from baseline compared to a 21% increase.
- Reductions in abnormal endoscopic findings and disease severity and extent (as measured at the microscopic level).
- Nominally significant improvement in the frequency and severity of EoE signs, and numerical reduction in days with at least one sign of EoE, based on caregiver-reported outcomes.
The safety results in the EoE KIDS study were generally consistent with the known safety profile of Dupixent in adolescents and adults with EoE. The most common adverse reactions for Dupixent overall are injection site reactions, conjunctivitis, conjunctivitis allergic, arthralgia, oral herpes and eosinophilia. In addition, the adverse reaction of injection site bruising was reported in EoE. In patients aged one to 11 years, adverse events more commonly observed with Dupixent (≥10%) in either weight-based dosing regimen compared to placebo during Part A were COVID-19, nausea, injection site pain, and headache. The long-term safety profile of Dupixent evaluated in Part B was similar to that observed during Part A.
George D. Yancopoulos, M.D., Ph.D.
Board co-Chair, President, and Chief Scientific Officer at Regeneron
“Eosinophilic esophagitis presents a unique challenge in young children, who struggle with their basic ability to eat during a time in their lives where proper nutrition is essential for growth and development. This approval will bring the proven efficacy and demonstrated safety profile of Dupixent to this vulnerable, young population that has already been established in older EoE patients and has the potential to transform the standard of care for children with EoE who previously had no therapies specifically approved for them.”
About EoE
EoE is a chronic, progressive disease associated with type-2 inflammation that is thought to be responsible for damaging the esophagus and impairing its function. Diagnosis is difficult, as symptoms can be mistaken for other conditions leading to delays in diagnosis. EoE can severely impact a child’s ability to eat and may also cause vomiting, abdominal pain, difficulty swallowing, decreased appetite, and challenges thriving. Continuous management of EoE may be needed to reduce the risk of complications and disease progression.
About the Dupixent pediatric EoE study
The EoE KIDS phase 3 study was a randomized, double-blind, placebo-controlled study evaluating the efficacy and safety of Dupixent in children aged one to 11 years with EoE. Part A enrolled 71 patients and evaluated Dupixent at a weight-based dose regimen, compared to placebo, for 16 weeks. Part B was a 36-week extended active treatment period in which eligible children from Part A in the Dupixent group continued treatment, while those in the placebo group switched to Dupixent. Patients included in this trial were previously treated and did not respond to conventional medicinal therapies, including proton pump inhibitors and/or swallowed topical corticosteroids.
The primary endpoint was histologic remission at 16 weeks, and secondary endpoints included assessments of endoscopic and histopathologic measures of the severity of disease along with caregiver-reported clinical signs and symptoms of EoE. The 108-week open-label extension period (Part C) to evaluate longer-term outcomes was recently completed.
Results from the study were published in The New England Journal of Medicine.
About Dupixent
Dupixent (dupilumab) is an injection administered under the skin (subcutaneous injection) at different injection sites. In patients aged one to 11 years with EoE, Dupixent is administered every other week (200 mg for children ≥15 to <30 kg, 300 mg for children ≥30 to <40 kg) or every week (300 mg for children ≥40 kg), based on weight. Dupixent is intended for use under the guidance of a healthcare professional and can be given in a clinic or at home administered by a caregiver after training by a healthcare professional.
Dupixent is a fully human monoclonal antibody that inhibits the signaling of the interleukin-4 (IL4) and interleukin-13 (IL13) pathways and is not an immunosuppressant. The Dupixent development program has shown significant clinical benefit and a decrease in type-2 inflammation in phase 3 studies, establishing that IL4 and IL13 are two of the key and central drivers of the type-2 inflammation that plays a major role in multiple related and often co-morbid diseases.
Dupixent has received regulatory approvals in more than 60 countries in one or more indications including certain patients with atopic dermatitis, asthma, chronic rhinosinusitis with nasal polyps, EoE, prurigo nodularis, chronic spontaneous urticaria, and chronic obstructive pulmonary disease in different age populations. More than 1,000,000 patients are being treated with Dupixent globally.
Dupilumab development program
Dupilumab is being jointly developed by Sanofi and Regeneron under a global collaboration agreement. To date, dupilumab has been studied across more than 60 clinical studies involving more than 10,000 patients with various chronic diseases driven in part by type-2 inflammation.
In addition to the currently approved indications, Sanofi and Regeneron are studying dupilumab in a broad range of diseases driven by type-2 inflammation or other allergic processes in phase 3 studies, including chronic pruritus of unknown origin and bullous pemphigoid. These potential uses of dupilumab are currently under clinical investigation, and the safety and efficacy in these conditions have not been fully evaluated by any regulatory authority.
About Regeneron
Regeneron REGN is a leading biotechnology company that invents, develops and commercializes life-transforming medicines for people with serious diseases. Founded and led by physician-scientists, our unique ability to repeatedly and consistently translate science into medicine has led to numerous approved treatments and product candidates in development, most of which were homegrown in our laboratories. Our medicines and pipeline are designed to help patients with eye diseases, allergic and inflammatory diseases, cancer, cardiovascular and metabolic diseases, neurological diseases, hematologic conditions, infectious diseases, and rare diseases.
Regeneron pushes the boundaries of scientific discovery and accelerates drug development using our proprietary technologies, such as VelociSuite®, which produces optimized fully human antibodies and new classes of bispecific antibodies. We are shaping the next frontier of medicine with data-powered insights from the Regeneron Genetics Center® and pioneering genetic medicine platforms, enabling us to identify innovative targets and complementary approaches to potentially treat or cure diseases.
For more information, please visit www.Regeneron.com or follow Regeneron on LinkedIn, Instagram, Facebook or X.
About Sanofi
We are an innovative global healthcare company, driven by one purpose: we chase the miracles of science to improve people’s lives. Our team, across the world, is dedicated to transforming the practice of medicine by working to turn the impossible into the possible. We provide potentially life-changing treatment options and life-saving vaccine protection to millions of people globally, while putting sustainability and social responsibility at the center of our ambitions.
Sanofi is listed on EURONEXT: SAN and NASDAQ: SNY
Sanofi Media Relations
Sandrine Guendoul | + 33 6 25 09 14 25 | sandrine.guendoul@sanofi.com
Evan Berland | + 1 215 432 0234 | evan.berland@sanofi.com
Victor Rouault | + 33 6 70 93 71 40 | victor.rouault@sanofi.com
Timothy Gilbert | + 1 516 521 2929 | timothy.gilbert@sanofi.com
Sanofi Investor Relations
Thomas Kudsk Larsen |+ 44 7545 513 693 | thomas.larsen@sanofi.com
Alizé Kaisserian | + 33 6 47 04 12 11 | alize.kaisserian@sanofi.com
Arnaud Delépine | + 33 6 73 69 36 93 |arnaud.delepine@sanofi.com
Felix Lauscher | + 1 908 612 7239 | felix.lauscher@sanofi.com
Keita Browne | + 1 781 249 1766 | keita.browne@sanofi.com
Nathalie Pham | + 33 7 85 93 30 17 | nathalie.pham@sanofi.com
Tarik Elgoutni | + 1 617 710 3587 | tarik.elgoutni@sanofi.com
Thibaud Châtelet | + 33 6 80 80 89 90 | thibaud.chatelet@sanofi.com
Regeneron Media Relations
Hannah Kwagh | +1 914-847-6314| hannah.kwagh@regeneron.com
Regeneron Investor Relations
Mark Hudson | + 914-847-3482 | mark.hudson@regeneron.com
Sanofi forward-looking statements
This press release contains forward-looking statements as defined in the Private Securities Litigation Reform Act of 1995, as amended. Forward-looking statements are statements that are not historical facts. These statements include projections and estimates regarding the marketing and other potential of the product, or regarding potential future revenues from the product. Forward-looking statements are generally identified by the words “expects”, “anticipates”, “believes”, “intends”, “estimates”, “plans” and similar expressions. Although Sanofi’s management believes that the expectations reflected in such forward-looking statements are reasonable, investors are cautioned that forward-looking information and statements are subject to various risks and uncertainties, many of which are difficult to predict and generally beyond the control of Sanofi, that could cause actual results and developments to differ materially from those expressed in, or implied or projected by, the forward-looking information and statements. These risks and uncertainties include among other things, unexpected regulatory actions or delays, or government regulation generally, that could affect the availability or commercial potential of the product, the fact that product may not be commercially successful, the uncertainties inherent in research and development, including future clinical data and analysis of existing clinical data relating to the product, including post marketing, unexpected safety, quality or manufacturing issues, competition in general, risks associated with intellectual property and any related future litigation and the ultimate outcome of such litigation, and volatile economic and market conditions, and the impact that pandemics or other global crises may have on us, our customers, suppliers, vendors, and other business partners, and the financial condition of any one of them, as well as on our employees and on the global economy as a whole. The risks and uncertainties also include the uncertainties discussed or identified in the public filings with the SEC and the AMF made by Sanofi, including those listed under “Risk Factors” and “Cautionary Statement Regarding Forward-Looking Statements” in Sanofi’s annual report on Form 20-F for the year ended December 31, 2023. Other than as required by applicable law, Sanofi does not undertake any obligation to update or revise any forward-looking information or statements.
All trademarks mentioned in this press release are the property of the Sanofi group apart from VelociSuite and Regeneron Genetics Center.
Regeneron Forward-Looking Statements and Use of Digital Media
This press release includes forward-looking statements that involve risks and uncertainties relating to future events and the future performance of Regeneron Pharmaceuticals, Inc. (“Regeneron” or the “Company”), and actual events or results may differ materially from these forward-looking statements. Words such as “anticipate,” “expect,” “intend,” “plan,” “believe,” “seek,” “estimate,” variations of such words, and similar expressions are intended to identify such forward-looking statements, although not all forward-looking statements contain these identifying words. These statements concern, and these risks and uncertainties include, among others, the nature, timing, and possible success and therapeutic applications of products marketed or otherwise commercialized by Regeneron and/or its collaborators or licensees (collectively, “Regeneron’s Products”) and product candidates being developed by Regeneron and/or its collaborators or licensees (collectively, “Regeneron’s Product Candidates”) and research and clinical programs now underway or planned, including without limitation Dupixent® (dupilumab) for the treatment of children aged 1 to 11 years with eosinophilic esophagitis; uncertainty of the utilization, market acceptance, and commercial success of Regeneron’s Products and Regeneron’s Product Candidates and the impact of studies (whether conducted by Regeneron or others and whether mandated or voluntary), including the studies discussed or referenced in this press release, on any of the foregoing; the likelihood, timing, and scope of possible regulatory approval and commercial launch of Regeneron’s Product Candidates and new indications for Regeneron’s Products, such as Dupixent for the treatment of chronic pruritus of unknown origin, bullous pemphigoid, and other potential indications; the ability of Regeneron’s collaborators, licensees, suppliers, or other third parties (as applicable) to perform manufacturing, filling, finishing, packaging, labeling, distribution, and other steps related to Regeneron’s Products and Regeneron’s Product Candidates; the ability of Regeneron to manage supply chains for multiple products and product candidates; safety issues resulting from the administration of Regeneron’s Products (such as Dupixent) and Regeneron’s Product Candidates in patients, including serious complications or side effects in connection with the use of Regeneron’s Products and Regeneron’s Product Candidates in clinical trials; determinations by regulatory and administrative governmental authorities which may delay or restrict Regeneron’s ability to continue to develop or commercialize Regeneron’s Products and Regeneron’s Product Candidates; ongoing regulatory obligations and oversight impacting Regeneron’s Products, research and clinical programs, and business, including those relating to patient privacy; the availability and extent of reimbursement of Regeneron’s Products from third-party payers, including private payer healthcare and insurance programs, health maintenance organizations, pharmacy benefit management companies, and government programs such as Medicare and Medicaid; coverage and reimbursement determinations by such payers and new policies and procedures adopted by such payers; competing drugs and product candidates that may be superior to, or more cost effective than, Regeneron’s Products and Regeneron’s Product Candidates; the extent to which the results from the research and development programs conducted by Regeneron and/or its collaborators or licensees may be replicated in other studies and/or lead to advancement of product candidates to clinical trials, therapeutic applications, or regulatory approval; unanticipated expenses; the costs of developing, producing, and selling products; the ability of Regeneron to meet any of its financial projections or guidance and changes to the assumptions underlying those projections or guidance; the potential for any license, collaboration, or supply agreement, including Regeneron’s agreements with Sanofi and Bayer (or their respective affiliated companies, as applicable) to be cancelled or terminated; the impact of public health outbreaks, epidemics, or pandemics (such as the COVID-19 pandemic) on Regeneron’s business; and risks associated with intellectual property of other parties and pending or future litigation relating thereto (including without limitation the patent litigation and other related proceedings relating to EYLEA® (aflibercept) Injection), other litigation and other proceedings and government investigations relating to the Company and/or its operations (including the pending civil proceedings initiated or joined by the U.S. Department of Justice and the U.S. Attorney’s Office for the District of Massachusetts), the ultimate outcome of any such proceedings and investigations, and the impact any of the foregoing may have on Regeneron’s business, prospects, operating results, and financial condition. A more complete description of these and other material risks can be found in Regeneron’s filings with the U.S. Securities and Exchange Commission, including its Form 10-K for the year ended December 31, 2023 and its Form 10-Q for the quarterly period ended September 30, 2024. Any forward-looking statements are made based on management’s current beliefs and judgment, and the reader is cautioned not to rely on any forward-looking statements made by Regeneron. Regeneron does not undertake any obligation to update (publicly or otherwise) any forward-looking statement, including without limitation any financial projection or guidance, whether as a result of new information, future events, or otherwise.
Regeneron uses its media and investor relations website and social media outlets to publish important information about the Company, including information that may be deemed material to investors. Financial and other information about Regeneron is routinely posted and is accessible on Regeneron’s media and investor relations website (https://investor.regeneron.com) and its LinkedIn page (https://www.linkedin.com/company/regeneron-pharmaceuticals).

© 2024 Benzinga.com. Benzinga does not provide investment advice. All rights reserved.
Ivans Index October 2024 Results Released
Milwaukee, WI., Nov. 05, 2024 (GLOBE NEWSWIRE) — Ivans® today announced the October 2024 results of the Ivans Index™, the insurance industry’s premium renewal rate index. Year over year, Commercial Auto, BOP, General Liability, Commercial Property and Umbrella experienced increasing average premium renewal rates, while premium renewal rates decreased for Workers’ Compensation. All major commercial lines premium renewal rates were lower month over month.
Premium renewal rate change by line of business for October 2024 highlights include:
- Commercial Auto: 9.62%, down slightly from 9.67% last month.
- BOP: 8.72%, down slightly from 8.76% at the end of September.
- General Liability: 3.89%, down from 4.48% the month prior.
- Commercial Property: 11.24%, down from 11.80% in September.
- Umbrella: 8.49%, down from 8.60% the month prior.
- Workers’ Compensation: -1.43%, down from -1.24% last month.
Released monthly, Ivans Index is a data-driven report of current conditions and trends for premium rate renewal change of the most placed commercial lines of business in the insurance industry. Analyzing more than 120 million data transactions, the Ivans Index premium renewal rate change measures the premium difference year over year for a single consistent policy. Inclusive of more than 38,000 agencies and 700 carriers and MGAs, the Ivans Index is reflective of the premium rate change trends being experienced by all agencies and insurers across the U.S. insurance market. Ivans Index is available to agencies and insurers as part of Market Insights at markets.ivansinsurance.com.
Download the complete Q3 Ivans Index report.
# # #
The Ivans logos are trademarks of Applied Systems, Inc., registered in the U.S.
About Ivans
Ivans is where insurance carriers, agents, and MGAs come together to grow their businesses. Every day, our 38,000 agents and over 700 carrier and MGA partners plug into technology that empowers them to better determine appetite and eligibility, swiftly produce quotes, get accurate claims and commission updates, automatically communicate policy data, and connect to one another to drive new business. With easier ways to get the day’s work done, insurance professionals can open the door to more revenue without letting complexity in behind it.

Lauren Malcolm Ivans 678-438-5093 lmalcolm@appliedsystems.com
Market News and Data brought to you by Benzinga APIs
© 2024 Benzinga.com. Benzinga does not provide investment advice. All rights reserved.
Lument Finance Trust Announces Quarter-End Earnings Release and Investor Call Dates
NEW YORK, Nov. 5, 2024 /PRNewswire/ — Lument Finance Trust, Inc. LFT (“we,”; “LFT” or “the Company”) announced today that it expects to file its Quarterly Report on Form 10-Q for the quarter ended September 30, 2024 with the Securities and Exchange Commission on Tuesday, November 12, 2024, after the market closes, and invites investors and other interested parties to listen to its live conference call via telephone or webcast on Wednesday, November 13, 2024 at 8:30 a.m. eastern time.
The conference call may be accessed by dialing 1-800-836-8184 (U.S.) or 1-646-357-8785 (international). Note: there is no passcode; please ask the operator to be joined into the Lument Finance Trust call. A live webcast, on a listen-only basis, is also available and can be accessed through the URL:
https://app.webinar.net/lDnbp3nxz36
For those unable to listen to the live broadcast, a recorded replay will be available by telephone dial-in. The replay call-in number is 1-888-660-6345 (U.S.) or 1-646-517-4150 (international) with passcode 01454.
About LFT
LFT is a Maryland corporation focused on investing in, financing and managing a portfolio of commercial real estate debt investments. The Company primarily invests in transitional floating rate commercial mortgage loans with an emphasis on middle-market multi-family assets. LFT is externally managed and advised by Lument Investment Management, a Delaware limited liability company.
Additional Information and Where to Find It
Investors, security holders and other interested persons may find additional information regarding the Company at the SEC’s Internet site at https://www.sec.gov/ or the Company website https://lumentfinancetrust.com or by directing requests to: Lument Finance Trust, 230 Park Avenue, 20th Floor, New York, NY 10169, Attention: Investor Relations.
Forward-Looking Statements
Certain statements included in this press release constitute forward-looking statements intended to qualify for the safe harbor contained in Section 27A of the Securities Act of 1933, as amended, and Section 21E of the Securities Exchange Act, as amended. Forward-looking statements are subject to risks and uncertainties. These forward-looking statements include information about possible or assumed future results of our business, financial condition, liquidity, results of operations, plans and objectives. You can identify forward-looking statements by use of words such as “believe,” “expect,” “anticipate,” “project,” “estimate,” “plan,” “continue,” “intend,” “should,” “may,” “will,” “seek,” “would,” “could,” or similar expressions or other comparable terms, or by discussions of strategy, plans or intentions. Forward-looking statements are based on our beliefs, assumptions and expectations of our future performance, taking into account all information currently available to us on the date of this press release or the date on which such statements are first made. Actual results may differ from expectations, estimates and projections. You are cautioned not to place undue reliance on forward-looking statements in this press release and should consider carefully the factors described in Part I, Item IA “Risk Factors” in the Company’s Annual Report on Form 10-K for the year ended December 31, 2023, which is available on the Securities and Exchange Commission’s (“SEC”) website at www.sec.gov, and in other current or periodic filings with the SEC, when evaluating these forward-looking statements. Forward-looking statements are subject to substantial risks and uncertainties, many of which are difficult to predict and are generally beyond our control. Except as required by applicable law, the Company disclaims any intention or obligation to update or revise any forward-looking statements, whether as a result of new information, future events or otherwise.
![]() View original content to download multimedia:https://www.prnewswire.com/news-releases/lument-finance-trust-announces-quarter-end-earnings-release-and-investor-call-dates-302296936.html
View original content to download multimedia:https://www.prnewswire.com/news-releases/lument-finance-trust-announces-quarter-end-earnings-release-and-investor-call-dates-302296936.html
SOURCE Lument Finance Trust, Inc.
Market News and Data brought to you by Benzinga APIs
© 2024 Benzinga.com. Benzinga does not provide investment advice. All rights reserved.
Bond Market Fear Gauge Soars To 1-Year High Ahead Of US Election Results: 'Bond Vigilantes Have Been Voting Early'
A widely watched gauge of bond market volatility has surged to its highest level in over a year as American voters cast their ballots in one of the closest presidential races in recent history.
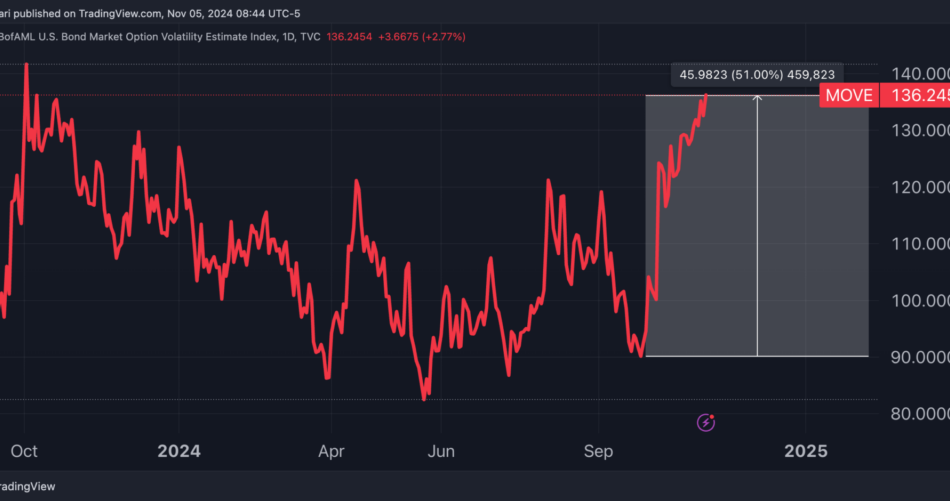
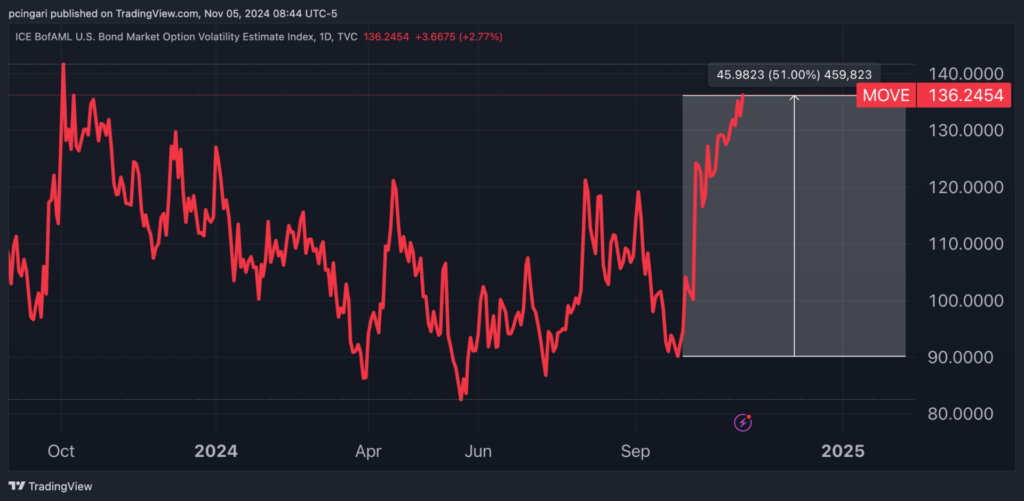
The ICE BofAML U.S. Bond Market Option Volatility Estimate Index, or “MOVE” index, closed at 136 on Monday, reaching levels last seen in early October 2023.
This spike in the MOVE index highlights mounting anxiety in the bond market, driven by political uncertainty and fears of inflationary fiscal policies.
What Is The MOVE Index?
The MOVE index, often described as the VIX for bonds, measures expected volatility in the Treasury market based on options pricing.
Like the VIX, which tracks volatility in the equity market, the MOVE index is seen as a “fear gauge” for fixed-income investors.
Higher MOVE readings indicate increased uncertainty about future bond price movements, reflecting traders’ fears around interest rates, inflation, and broader economic conditions.
See Also: Beginners Guide to VIX or Volatility Indices
Election Jitters Fuel Bond Market Anxiety
The U.S. presidential election has investors on edge, with polls showing one of the tightest races in modern American history.
Neither Vice President Kamala Harris nor ex-President Donald Trump held a meaningful lead in critical battleground states ahead of Nov. 5, wrote Nate Cohn, chief political analyst at the New York Times.
“In the history of modern polling, there’s never been a campaign where the final polls showed such a close contest,” he added.
The possibility of a prolonged, contentious result has amplified concerns in the bond market, where volatility has jumped 51% since late September.
Fixed-income investors are also weighing the potential fiscal impact of each candidate’s policies, especially if the winner’s administration fuels inflation and adds to the federal debt.
According to the Committee for a Responsible Federal Budget (CRFB), Trump’s spending plans could increase the national debt by $7.75 trillion over the next decade, driving the debt-to-GDP ratio to 143% by 2035. By contrast, Kamala Harris’s proposals would add $3.95 trillion to the debt, resulting in a debt-to-GDP ratio of approximately 134% by the same year.
Chart: Bond Volatility Index Has Risen By Over 50% Since Late-September
Trump’s Tariff Plans Stoke Inflation Fears
Under a Trump administration, inflation could rise if proposed tariffs on Chinese imports and other goods come into effect.
Trump has floated a 60% tariff on Chinese products and a universal 10% tariff, policies which JPMorgan analysts estimate could drive inflation up by as much as 2.4% in a worst-case scenario.
“Tariffs are inflationary,” IMF deputy managing director Gita Gopinath warned in October.
With inflation a growing concern, Treasury yields have been on an upward trajectory, as investors demand higher returns to offset potential future price increases.
Yields on the 10-year Treasury bond have risen by 50 basis points last month and hit 4.33% on Tuesday.
‘Bond Vigilantes’ Are Back
The bond market’s reaction to these uncertainties has been swift and severe.
“Nothing seems to be stopping U.S. Treasury bond yields from rising,” said veteran Wall Street investor Ed Yardeni.
“The Bond Vigilantes have been voting early (and voting often) by selling US Treasury notes and bonds,” he added.
The iShares 20+ Year Treasury Bond ETF TLT, which tracks long-dated Treasuries, fell nearly 6% in October 2024.
With both candidates likely to enact substantial stimulus, Yardeni stated, “The bond market is tightening the economy itself,” as investors anticipate inflation and higher interest rates.
Yardeni indicated this surge in yields could push the 10-year Treasury into the 5% range, a level not seen since before the financial crisis.
Now Read:
Image: Shutterstock
© 2024 Benzinga.com. Benzinga does not provide investment advice. All rights reserved.
Cast Iron Cookware Market Size Forecasted at USD 2.2 Billion by 2031, Rising at 3.7% CAGR with Demand for Healthy Cooking Solutions Driving Growth| Transparency Market Research, Inc.
Wilmington, Delaware, United States, Transparency Market Research Inc. , Nov. 05, 2024 (GLOBE NEWSWIRE) — The global cast iron cookware industry was valued at US$ 1.6 billion in 2022. A CAGR of 3.7% is expected from 2023 to 2031, reaching US$ 2.2 billion during the forecast period. Iron is particularly beneficial to individuals who suffer from iron deficiency when foods are cooked in cast iron. Researchers have found that iron contained in cookware enters food through the cooking process, similar to the iron found in vegetables. Hence, this helps prevent anemia by increasing iron intake.
Sustainable and eco-friendly cookware may become more popular as environmental concerns gain importance. Manufacturers may explore more environmentally friendly production processes based on the durability and long lifespan of cast iron. As consumers’ needs evolve, manufacturers may be able to create new styles, shapes, and features by using new technology or design. Heat distribution could be improved, ergonomic handles could be introduced, and other improvements could be made to facilitate user use.
Download Sample of the Report: https://www.transparencymarketresearch.com/sample/sample.php?flag=S&rep_id=40196
Small-batch and handmade cast iron cookware are often purchased by consumers who are willing to pay a premium for them. Investing in innovative design elements, like modern shapes, preseasoned options, and improved handles, also plays an important role in the market’s growth.

Global Cast Iron Cookware Market: Key Players
Leading manufacturers invest in research and development, expand their products, and acquire other companies. Product development is a top priority for top players. There are a large number of regional and global players on the market, making it highly competitive.
- American Metalcraft, Inc.
- Camp Chef, Inc.
- FINEX Cast Iron Cookware Co.
- Lava Cookware USA
- Lodge Manufacturing Company
- Made In
- Marquette Castings
- Meyer Corporation
- The Coleman Company, Inc.
- Tramontina USA, Inc.
- Other Key Players
Key Findings of the Market Report
- Based on type, the enamel-coated segment is expected to drive demand for cast iron cookware.
- In terms of style, Dutch ovens are likely to drive the cast iron cookware market.
- A significant portion of the growth in demand will be driven by the food services industry.
- Supermarkets/hypermarkets are likely to drive the cast iron cookware market.
- Asia Pacific will hold a significant share during the forecast period.
- North America is predicted to grow fastest in the cast iron cookware market.
Global Cast Iron Cookware Market: Growth Drivers
- The durability and long life of cast iron cookware make it an excellent choice. In addition to being long-lasting, cast iron cookware is affordable, making it an excellent investment. In addition to being versatile, cast iron cookware can be used for many different cooking methods. Those seeking multi-functional kitchen goods will appreciate the versatility of these products.
- Cast iron cookware is popular due to its vintage or retro aesthetic. Decorative and functional purposes can both be served by cast iron pieces due to their classic, timeless look. Sustainable and eco-friendly products are growing in popularity as consumers become more environmentally conscious. Due to its durability and low maintenance, cast iron cookware fits perfectly with this style of cooking.
- In recent years, celebrity chefs, cooking shows, and culinary trends have contributed to an increase in awareness of premium cookware, including cast iron. Consumer preferences are influenced by endorsements from chefs and influencers. Artisanal and locally crafted products are becoming increasingly popular.
Get Sample PDF Research Report with Latest Industry Insights: https://www.transparencymarketresearch.com/sample/sample.php?flag=S&rep_id=40196
Global Cast Iron Cookware Market: APAC to be the Go-To Investment Destination
- The Asia Pacific region is expected to lead the market for cast iron cookware market. Demand for cast iron cookware is expected to rise in the coming years due to the rise in the usage of cast iron cookware in developing countries such as India and China. Several Asia-Pacific countries have experienced economic growth that has increased disposable income. With people’s changing lifestyles and their desire for premium, durable kitchenware, cast iron cookware has become increasingly popular.
- In the Asia Pacific, cast iron cooking may offer health benefits. In addition, Western culinary trends have gained popularity in Asia-Pacific and cast iron cookware is becoming more popular. Cultural exchange and tourism have contributed to the introduction of new techniques and utensils in the kitchen. Tourists and expats, influencing local tastes, may bring the appreciation of cast iron cookware back.
Key Developments
- In April 2023, Meyer Corporation’s Rachael Ray brand launched Nitro Cast Iron, a cast iron collection with a protective enamel that prevents chipping, rusting, and staining.
- In September 2023, Stove Kraft Ltd., a leading global manufacturer of cast iron cookware, opened the world’s first factory used for casting iron. By April 2024, the company will start production trials at this foundry, and products from it will be on the market.
Global Cast Iron Cookware Market: Segmentation
By Product Type
- Unseasoned
- Seasoned
- Enamel Coated
By Style
- Dutch Ovens
- Camp Pots
- Skillets/Fryers
- Woks
- Others (Griddles etc.)
By End User
By Distribution Channel
- Company-owned websites
- E-commerce websites
- Supermarkets/Hypermarkets
- Specialty Stores
- Others (Retail Stores etc.)
By Region
- North America
- Europe
- Asia Pacific
- Middle East & Africa
- South America
Buy this Premium Research Report: https://www.transparencymarketresearch.com/checkout.php?rep_id=40196<ype=S
More Trending Report by Transparency Market Research:
- Hair Removal Products Market – The global hair removal products market (marché des produits d’épilation) is expected to grow at a CAGR of 5.9% from 2021 to 2031. By the end of 2031, the global hair removal products market is expected to exceed a valuation of US$ 1.8 Bn.
- Jet Hand Dryer Market – The global jet hand dryer market (marché des sèche-mains à jet) stood at US$ 443.4 million in 2022 and the global market is projected to reach US$ 1.0 billion by 2031. The global industry is anticipated to expand at a CAGR of 9.9% between 2023 and 2031.
- Europe External Blinds Market – The Europe external blinds market (marché des stores extérieurs) is estimated to grow at a CAGR of 3.0% from 2024 to 2034 and reach US$ 35.9 Billion by the end of 2034.
- Solar Cooker Market – The global Solar Cooker Market is estimated to grow at a CAGR of 5.4% from 2023 to 2031 and reach US$ 3.5 Billion by the end of 2031.
About Transparency Market Research
Transparency Market Research, a global market research company registered at Wilmington, Delaware, United States, provides custom research and consulting services. Our exclusive blend of quantitative forecasting and trends analysis provides forward-looking insights for thousands of decision makers. Our experienced team of Analysts, Researchers, and Consultants use proprietary data sources and various tools & techniques to gather and analyses information.
Our data repository is continuously updated and revised by a team of research experts, so that it always reflects the latest trends and information. With a broad research and analysis capability, Transparency Market Research employs rigorous primary and secondary research techniques in developing distinctive data sets and research material for business reports.
Contact:
Transparency Market Research Inc.
CORPORATE HEADQUARTER DOWNTOWN,
1000 N. West Street,
Suite 1200, Wilmington, Delaware 19801 USA
Tel: +1-518-618-1030
USA – Canada Toll Free: 866-552-3453
Website: https://www.transparencymarketresearch.com
Email: sales@transparencymarketresearch.com
Follow Us: LinkedIn| Twitter| Blog | YouTube

Market News and Data brought to you by Benzinga APIs
© 2024 Benzinga.com. Benzinga does not provide investment advice. All rights reserved.